How to Find the Most Acidic Proton in a Molecule
Therefore p should be the most acidic. Hydrogens directly attached to very electronegative atoms such as oxygen sulphur and the halogens carry a substantial degree of acidity.

How To Find The Most Acidic H In A Molecule Youtube
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.

. -ОН OH но Но Click on the most acidic proton in the molecule below. Check all that apply. Acidic protons are usually bound to O or N.
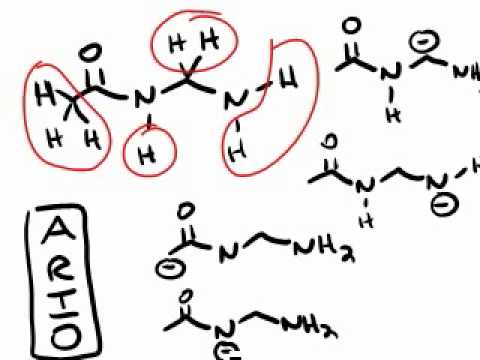
The numbers represent potential starting or ending places for arrows represents a lone pair on nitrogen 2 represents the hydrogen. Who are the experts. This means the most acidic proton in this molecule is the on the terminal alkyne sp C-H.
12 H2SeO3 has a p𝐾a of 264HSeO3 has ap𝐾aof 828. The following guidelines can be used to predict acidity. Y is an alkyl proton para to a carbonyl.
Click on the most acidic proton in the molecule below. Protons on C1 are numbered 1 through 3 protons on C3 are numbered 4. The most acidic proton is the one that upon removal will yield the most stable conjugate base.
The higher the value of the dissociation constant of acid K a the smaller is the p K a value and the more acidic the compound is. The test is four hours and 30 minutes long and contains four sectionsThe test is designed to assess your knowledge in. Therefore the first step is to look for all OH and NH bonds.
Experts are tested by Chegg as specialists in their subject area. HCl is a hydrogen halide with a pKa range of 3 to -10 and NH3 is most similar to a 1 amine with R H that would have an approximate pKa of 35. A H2SeO3 has more electron withdrawing groups thanHSeO3 B HSeO3 has fewer acidic protons than.
Its conjugate base is the weakest base here and is thus the most. Proton a is the most acidic. Hexane-24-dione molecule with protons numbered as follows according to IUPAC nomenclature.
X is an alkyl proton adjacent to a carbonyl. P is an amide proton. Examination of a pKa table reveals some trends for acidic protons.
So HCl would be the stronger acid. Scan and rank sounds simple but it conceals several difficulties that are elaborated below. Using resonance select the most acidic protons in the hexane-24-dione molecule.
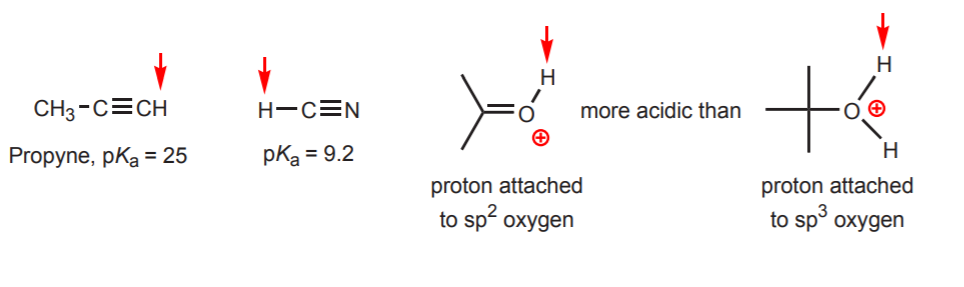
H2S also has a proton attached to an. The hydrocarbons are generally considered very weak acids but among them the alkynes with a pKa 25 are quite acidic. For example how could I tell which was the most acidic in.
I just need some clarification on how you decide which proton in a molecule is the most acidic as the explanation in my Organic Chemistry textbook isnt as clear as I would have hoped. HO OH NH2 Consider the below acid-base reaction. 11 Identify the most acidic proton in the following molecule.
The most acidic functional group usually is holding the most acidic H in the entire molecule. Which of the following statementsbest explains this difference. A A B B C C D D E EB A C D E.
Proton b is bonded to a more electronegative atom S. Directory of Chem Help ASAP videos. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Identify familiar functional groups. DO NOT FORGET TO SUBSCRIBEThis video illustrates how to determine the most acidic proton as well as how to determine which molecule is more acidic based on. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
This problem has been solved. To explain the p K a values we check for the stability of conjugate base which is obtained by removing the most acidic hydrogen and here in this case the conjugate base of the carboxylic acid will be most stable due to resonance. Hydrogens attached to a positively charged nitrogen oxygen or sulfur are acidic.
Chemistry questions and answers. Identify the most acidic proton in the molecule and provide a brief explanation for your answer. Im guessing its the hydrogens on the left methyl group because this would produce the largest negative charge on.
The Dental Admission Test DAT is a test administered by the American Dental Association ADA. It is not one of the functional groups in the list above but there is a similar proton is Table of acids that we learned. Scan a molecule for known acidic functional groups.
Z is an amine proton. For HCl the proton is attached to a more electronegative atom Cl than in NH3 where the proton is attached to an N. We review their content and use your feedback to keep the quality high.
Biology chemistry organic chemistry perceptual ability reading comprehension and basic math.
How To Identify The Most Acidic Proton
Ch 2 Ohv Identifying The Most Acidic Proton In A Molecule Youtube

Comments
Post a Comment